Sponsored by Glaukos
Minimally-invasive glaucoma management gets a major upgrade. Meet the iStent infinite®.
Nothing pairs better with lunch than a fresh perspective on glaucoma, and at ESCRS 2025, Glaukos served up both. The occasion? The much anticipated European launch of the iStent infinite®.
Over sandwiches and stents, a lineup of international experts challenged the “drops until you drop” playbook, spotlighted safety data and unveiled the latest clinical and real-world evidence. Through the hour-long session, one thing was clear: glaucoma management is stepping boldly into its interventional era, and the iStent infinite® is here to make the leap not only effective, but exceptionally safe.

Rethinking the glaucoma treatment paradigm
Dr. Sev Teymoorian (United States) kicked off the symposium by putting the brakes on business as usual in glaucoma management. For decades, the field has leaned heavily on the “meds-first and always” approach—escalating drop medications until surgery finally staggers onto the stage.
“The conventional pathway that we use—using some drops, having some more drops, eventually rolling up our sleeves and doing maybe glaucoma surgery—doesn’t work,” Dr. Teymoorian declared.

Add to that compliance, or rather, the lack of it. “Over 90% of patients are non-compliant to their drops,” he said, before delivering a stinger: “half of glaucoma patients are lost to follow-up in one year and two-thirds of them never return.”1-4
It’s this grim reality that’s fueling a shift toward what Dr. Teymoorian calls “interventional glaucoma,” a move from reaction to action, where earlier procedures step in before the optic nerve waves a white flag.
“Don’t wait for there to be nerve damage before we respond,” he advised attendees. “Don’t hope and cross your fingers that adding a few more eye drops will get the patient to good functional vision at the end of their lives.”
Safety first
Dr. Kevin Gillmann (Switzerland) took the stage armed with data—lots of it. His deep dive into MIGS safety pulled from more than 400 articles and an eye-watering (almost) 70,000 eye-years of patient observation.5
“As these procedures are increasingly offered early in the disease course to otherwise healthy patients, the key criterion behind the choice of these procedures has started to shift towards the safety of these techniques,” Dr. Gillmann explained.
His review sorted MIGS complications neatly into three buckets: your run-of-the-mill, short-lived annoyances (think IOP spikes), device-specific hiccups and the more worrisome long-term effects.
And things got especially interesting when he compared endothelial cell safety.

Drawing on a study by Dr. Ike Ahmed, Dr. Gillmann pointed out that the Hydrus (Ivantis, Inc.; CA, United States) looked fine at first glance, showing a non-statistically significant endothelial cell loss (ECL) at three months compared to cataract surgery alone. The catch? “As time goes by, the difference between the two curves widens, and it becomes statistically significant,” he noted.
By contrast, the iStent came off looking positively boring in the best possible way. “The two curves corresponding to the iStent and the phaco control… overlap. So here, no difference in the iStent,” Dr. Gillmann reported.
His bottom line? Of all the MIGS options, the iStent came out with “the most favorable safety profile.”
Next-generation injector
Dr. Imran Masood (United Kingdom) introduced the audience to the iStent infinite injector system, calling it “a step up [from the iStent inject® W] in terms of efficacy and also in terms of efficiency and predictability.” In other words, a slicker, sharper tool designed to make life easier for both surgeon and patient.
The device comes loaded with clever features:
- Stent delivery button that offers “an infinite number of clicks”
- Singulator that primes each stent for perfect deployment.
- 8° angled insertion tube…just enough tilt to dodge incision interference and give wide, easy access across the canal.
- Auto-retracting introducer tip for smooth entry, while keeping viscoelastic from sneaking out and ensuring the chamber stays nice and firm.
- Three wide-flange stents, shaped anatomically, channeling fluid flow through 240° of Schlemm’s canal.
“Having this angulation actually allows you to approach the trabecular meshwork in this sort of on-fast perpendicular approach that you need to get that stent in the right place within the canal,” Dr. Masood explained.
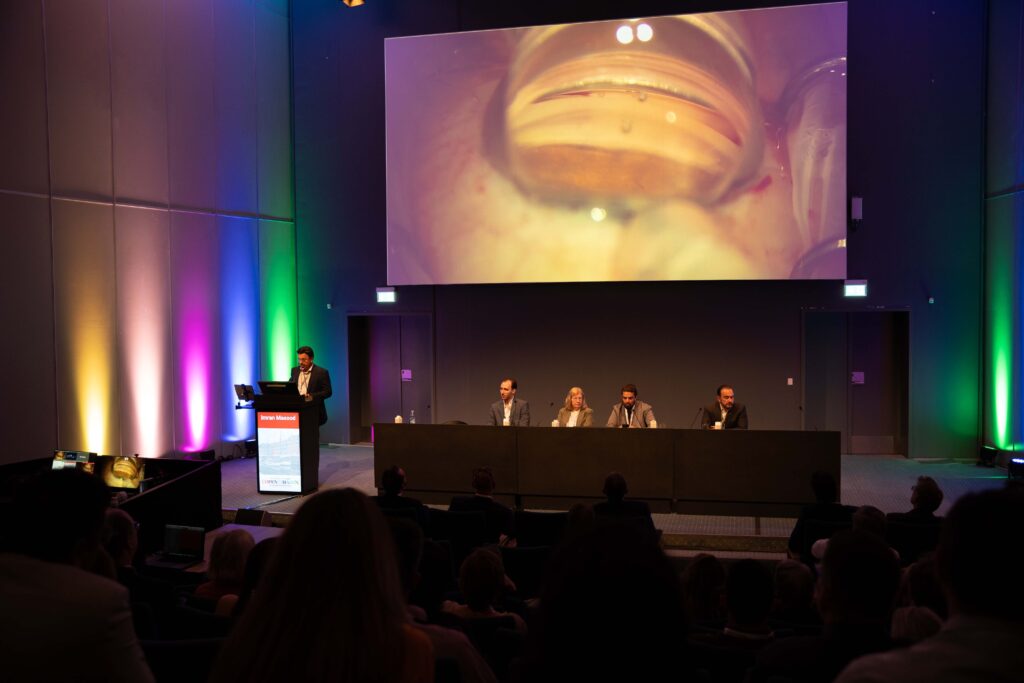
And then there’s the improved trocar visibility. According to Dr. Masood, “You can have this sort of view down the trocar almost like a snooker cue, so you know exactly where you are in terms of implanting accurately.” For anyone who’s ever lined up a tricky shot, the analogy landed perfectly.
To top it off, Dr. Masood shared videos from his first three cases, showcasing what he dubbed the “triple waterfall sign”—blood refluxing through all three stents, confirming both placement and function with a flourish.
Backed by clinical evidence…
Prof. Henny Beckers (The Netherlands) walked the audience through data from not one but two major studies on the iStent infinite®. The first was a pivotal trial including the toughest of the tough: patients who had either failed previous glaucoma surgery or maxed out on tolerated medical therapy.
“I was really surprised to see that the failed surgery group could get this good results with 73.4% having success6,” Prof. Beckers said. Even better, “31% of patients in the failed surgery subgroup reach IOPs of 15 [mmHg] and lower.6” Not bad for a group most would consider out of options.

Next up was the INTEGRITY trial, which pitted the iStent infinite® head-to-head against the trabecular bypass stent Hydrus (Ivantis, Inc.; CA, United States) in standalone procedures with medication washout.7 The verdict? “A statistically different outcome between iStent infinite® and the Hydrus group with a 13.2% difference,” Prof. Beckers reported, referring to the proportion of eyes that not only avoided surgical complications but also achieved an unmedicated IOP reduction of at least 20% from baseline.
The safety data also made for interesting reading: adverse events were lower with iStent infinite® (24.2% vs. 36.0%) and surgical complications were dramatically fewer (3.3% vs. 16.9%).7
…And real-world results
Dr. Ike Ahmed (Canada) shared real-world results from his practice using the iStent infinite® in tandem with phacoemulsification.His patient pool was far from straightforward—many had advanced disease—yet the numbers spoke clearly: mean IOP dropped from 15.9 to 13.4 mmHg, and medications nearly halved from 1.7 to 0.8.

“We had about 87.7% of patients with primary success at one year,” Dr. Ahmed reported. The real headline? Nothing went backwards. “No eyes had an increase in their IOP or increase in their medication count,” Dr. Ahmed said, adding that stent implantation came with zero intraoperative complications.
He also underlined a critical takeaway: timing matters. “Earlier intervention like MIGS or even SLT works better when we do it earlier in disease,” Dr. Ahmed advised. “If you leave a procedure like MIGS later on…when the disease is more fibrotic, irreversible, with more secondary changes in the outflow system and more optic nerve disease, they don’t work as well.” In other words, when it comes to glaucoma, waiting politely is not a winning strategy.
The takeaway
The session made one thing abundantly clear: the iStent infinite® isn’t just another incremental update. It’s a thoughtfully engineered tool poised to redefine MIGS. With its three-stent design covering up to 240° of Schlemm’s canal, a next-generation injector and a standout safety profile, it offers European ophthalmologists a compelling option for patients with moderate to advanced disease.
For those in the audience getting their first iStent infinite® experience, the symposium provided both practical guidance and scientific rationale, showing how the procedure can be integrated into treatment algorithms—especially for patients who might benefit from earlier surgical intervention or those who have exhausted prior options.
In short, the iStent infinite® represents more than a new device launched at ESCRS 2025. It signals a bold step forward in proactive, interventional glaucoma care, combining evidence, innovation and real-world results to help clinicians elevate outcomes for their patients.
Editor’s Note: The 43rd Congress of the European Society of Cataract and Refractive Surgeons (ESCRS 2025) is being held from 12-16 September in Copenhagen, Denmark. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all countries.
References
- Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598-606.
- Analysis of Market, Claims, and Compliance Data. Data on file. Glaukos Corporation.
- Sarkisian SR Jr, Ang RE, Lee AM, et al; GC-010 Travoprost Intraocular Implant Investigators. Phase 3 Randomized Clinical Trial of the Safety and Efficacy of Travoprost Intraocular Implant in Patients with Open-Angle Glaucoma or Ocular Hypertension. Ophthalmology. 2024;131(9):1021-1032.
- Sarkisian SR, Ang RE, Lee AM, et al. Travoprost Intracameral Implant for Open-Angle Glaucoma or Ocular Hypertension: 12-Month Results of a Randomized, Double-Masked Trial. Ophthalmol Ther. 2024;13(4):995-1014.
- Data on file. Manuscript currently submitted by Kevin Gillmann & Athena Lallouette
- Sarkisian SR Jr, Grover DS, Gallardo MJ, et al; iStent infinite Study Group. Effectiveness and Safety of iStent Infinite Trabecular Micro-Bypass for Uncontrolled Glaucoma. J Glaucoma. 2023;32(1):9-18.
- Ahmed IIK, Berdahl JP, Yadgarov A, et al. Six-Month Outcomes from a Prospective, Randomized Study of iStent infinite Versus Hydrus in Open-Angle Glaucoma: The INTEGRITY Study. Ophthalmol Ther. 2025;14(5):1005-1024.
iStent® IMPORTANT SAFETY INFORMATION
INDICATIONS FOR USE: The iStent® Trabecular Micro-Bypass System is intended to reduce intraocular pressure safely and effectively in patients diagnosed with primary open-angle glaucoma, pseudoexfoliative glaucoma or pigmentary glaucoma. The device is safe and effective when implanted in combination with cataract surgery in those subjects who require intraocular pressure reduction and/or would benefit from glaucoma medication reduction. The device may also be implanted in patients who continue to have elevated intraocular pressure despite prior treatment with glaucoma medications and conventional glaucoma surgery.
CONTRAINDICATIONS: The iStent® Trabecular Micro-Bypass Stent is contraindicated under the following circumstances or conditions: • In eyes with primary angle closure glaucoma, or secondary angle-closure glaucoma, including neovascular glaucoma, because the device would not be expected to work in such situations • In patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure.
WARNINGS/PRECAUTIONS: For prescription use only. This device has not been studied in patients with uveitic glaucoma. Do not use the devices if the Tyvek® lid has been opened or the packaging appears damaged. In such cases, the sterility of the device may be compromised. iStent is MR-Conditional. Physician training by Glaukos personnel is required prior to use of this device. Do not re-use the stent(s) or inserter, as this may result in infection and/or intraocular inflammation, as well as occurrence of potential postoperative adverse events. There are no known compatibility issues with the iStent and other intraoperative devices (e.g., viscoelastics) or glaucoma medications. Unused product & packaging may be disposed of in accordance with facility procedures. Implanted medical devices and infected products must be disposed of as medical waste.
iStent inject® W IMPORTANT SAFETY INFORMATION
INDICATIONS FOR USE: The iStent inject® W, is intended to reduce intraocular pressure safely and effectively in patients diagnosed with primary openangle glaucoma, pseudo-exfoliative glaucoma or pigmentary glaucoma. The iStent inject® W, can deliver two (2) stents on a single pass, through a single incision. The implant is designed to stent open a passage through the trabecular meshwork to allow for an increase in the facility of outflow and a subsequent reduction in intraocular pressure. The device is safe and effective when implanted in combination with cataract surgery in those subjects who require intraocular pressure reduction and/or would benefit from glaucoma medication reduction. The device may also be implanted in patients who continue to have elevated intraocular pressure despite prior treatment with glaucoma medications and conventional glaucoma surgery. CONTRAINDICATIONS: The iStent inject® W System is contraindicated under the following circumstances or conditions: • In eyes with primary angle closure glaucoma, or secondary angle closure glaucoma, including neovascular glaucoma, because the device would not be expected to work in such situations. • In patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure.
WARNINGS/PRECAUTIONS: • For prescription use only. • This device has not been studied in patients with uveitic glaucoma. • Do not use the device if the Tyvek® lid has been opened or the packaging appears damaged. In such cases, the sterility of the device may be compromised. • Due to the sharpness of certain injector components (i.e. the insertion sleeve and trocar), care should be exercised to grasp the injector body. Dispose of device in a sharps container. • iStent inject® W is MR Conditional. • Physician training is required prior to use of the iStent inject® W System. • Do not re-use the stent(s) or injector, as this may result in infection and/or intraocular inflammation, as well as occurrence of potential postoperative adverse events. • There are no known compatibility issues with the iStent inject® W and other intraoperative devices. (e.g., viscoelastics) or glaucoma medications. • Unused product & packaging may be disposed of in accordance with facility procedures. Implanted medical devices and contaminated products must be disposed of as medical waste. • The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. If intraocular pressure is not adequately maintained after surgery, the surgeon should consider an appropriate treatment regimen to reduce intraocular pressure. • Patients should be informed that placement of the stents, without concomitant cataract surgery in phakic patients, can enhance the formation or progression of cataract.
ADVERSE EVENTS: Please refer to Directions For Use for additional adverse event information. CAUTION: Please reference the Directions For Use labelling for a complete list of contraindications, warnings and adverse events.
iStent infinite® IMPORTANT SAFETY INFORMATION
INDICATION FOR USE: The iStent infinite System is intended to reduce intraocular pressure safely and effectively in adult patients diagnosed with primary open-angle glaucoma, pseudoexfoliative glaucoma or pigmentary glaucoma. The device is safe and effective when implanted in combination with or without cataract surgery in those subjects who require intraocular pressure reduction and/or would benefit from glaucoma medication reduction. The device may also be implanted in patients who continue to have elevated intraocular pressure despite prior treatment with glaucoma medications and/or conventional glaucoma surgery.
CONTRAINDICATIONS: The iStent infinite System is contraindicated under the following circumstances or conditions: •In eyes with primary angle closure glaucoma, or secondary angle-closure glaucoma, including neovascular glaucoma, because the device would not be expected to work in such situations.• In patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure.
WARNINGS/PRECAUTIONS: •For prescription use only. • Intended users are trained ophthalmologists only. • This device has not been studied in patients with uveitic glaucoma. • Do not use the device if the Tyvek® lid has been opened or the packaging appears damaged. In such cases, the sterility of the device may be compromised. • Due to the sharpness of certain injector components (i.e., the insertion sleeve and trocar), care should be exercised to grasp the injector body. Dispose of device in a sharps container. • iStent infinite is MR-Conditional • Physician training is required prior to use of the iStent infinite System. • Do not re-use the stent(s) or injector, as this may result in infection and/or intraocular inflammation, as well as occurrence of potential postoperative adverse events • There are no known compatibility issues with theiStent infinite and other intraoperative devices (e.g., viscoelastics) or glaucoma medications. • Unused product & packaging may be disposed of in accordance with facility procedures. Implanted medical devices and contaminated products must be disposed of as medical waste. • The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. If intraocular pressure is not adequately maintained after surgery, the surgeon should consider an appropriate treatment regimen to reduce intraocular pressure. • Patients should be informed that placement of the stents, without concomitant cataract surgery in phakic patients, can enhance the formation or progression of cataract. ADVERSE EVENTS: The most common postoperative adverse events reported in the iStent infinite pivotal trial included IOP increase ≥ 10 mmHg vs. baseline IOP (8.2%), loss of BSCVA ≥ 2 lines (11.5%), ocular surface disease (11.5%), perioperative inflammation (6.6%) and visual field loss ≥ 2.5 dB (6.6%). CAUTION: Please see DFU for a complete list of contraindications, warnings, precautions, and adverse events.
Glaukos®, iStent®, iStent inject® W and iStent infinite® are registered trademarks of Glaukos Corporation.
All rights reserved. ©2025. PM-EU-0376; PM-EU-0377; PM-EU-0381; PM-EU-0388

