The nod for injecting viscoelastic during ophthalmic procedures will be immediately followed by a US market launch.
New World Medical (Rancho Cucamonga, California, United States) announced on Feb 18 that the FDA has granted 501(k) clearance to its VIA360 Surgical System.
The nod has come for the injection of controlled amounts of viscoelastic fluid during ophthalmic surgeries, including standalone glaucoma procedures or those combined with cataract surgeries. The company stated that the device is already indicated to cut the trabecular meshwork tissue during trabeculotomies.
“The clearance of VIA360 marks another milestone in our mission to deliver innovative solutions that empower surgeons and improve the lives of patients globally,” said Khalid Mansour, president and COO of New World Medical in a press release.
“This system was developed in close collaboration with ophthalmic surgeons, addressing key challenges in surgery and providing greater control, precision and flexibility.”
VIA360’s key features
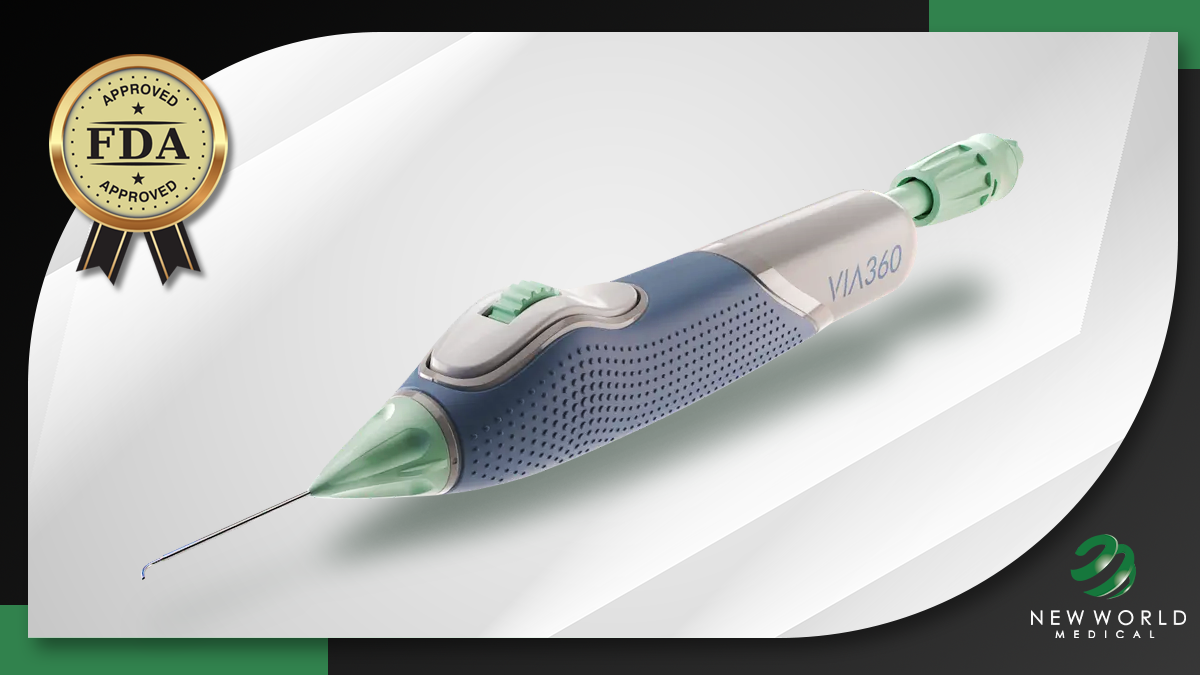
New World has highlighted the device’s proprietary ActiveInject Technology, which it claims offers catheter movement-independent viscoelastic delivery, and its Smart Prime System, which is designed to offer optimized viscoelastic efficiency delivery.
Another key design feature is VIVA360’s microcatheter, so named because of its ability to advance up to 360 degrees with microchannels. According to the company, this allows targeted forward and tangential delivery of viscoelastic fluid.
VIVA 360 also features a rotatable cannula, which has advantages in positioning and flexibility for a single entry without full device rotation.
Renowned American glaucoma surgeon Dr. I. Paul Singh, president of the Eye Centers of Racine & Kenosha in Wisconsin, commented on the advantages in precision and control this offers to surgeons and the resultant patient outcomes.
“The VIA360 facilitates a targeted approach that is tailored to each patient’s individual needs,” he said. “The technology elevates the efficiency of the procedure by allowing me to deliver viscoelastic exactly where and when it’s needed, with complete control at my fingertips.”
Coming to America?
Along with the announcement, New World has indicated that it won’t waste any time getting VIA360 into the hands of surgeons.
The company plans to roll out the VIA360™ immediately in the United States market, though pricing details haven’t been disclosed.
Editor’s Note: This content is intended exclusively for healthcare professionals and eye care practitioners. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.