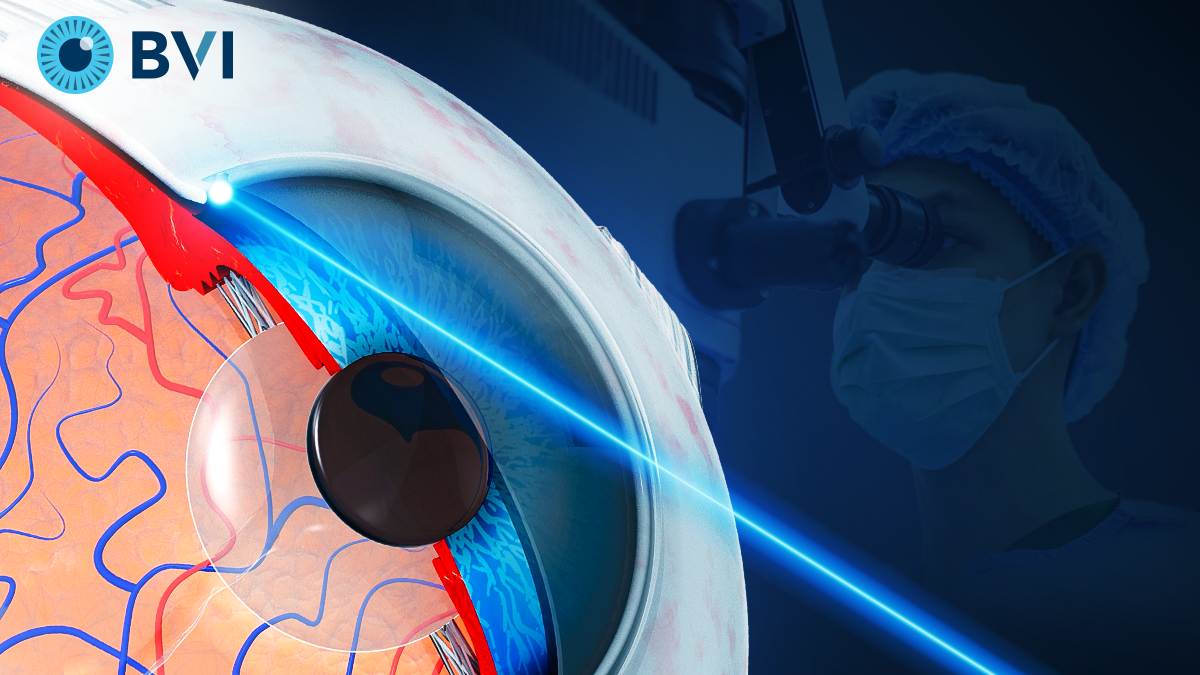
The FDA gave 510(k) clearance for BVI’s laser ECP platform, which offers minimally invasive, endoscopic precision for IOP control.
On April 17, BVI Medical (Massachusetts, United States) received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its laser endoscopy ophthalmic system (Leos), a novel glaucoma surgical platform that integrates laser technology with real-time endoscopic visualization.
BVI Medical announces the news on its LinkedIn page.
“This momentous milestone underscores our commitment to innovation,” said Shervin Korangy, president and CEO of BVI Medical, in a news release. “We have been building a strong pipeline that is starting to play out in numerous geographies across the world, including Leos in the U.S. market.”
READ MORE: A New Interventional Glaucoma Consensus Protocol Has Arrived
The Leos system is designed to lower intraocular pressure (IOP) by targeting aqueous humor production via a minimally invasive ab interno approach. According to BVI, it is the only FDA-cleared laser platform that combines endoscopic capabilities with a laser-based procedure to perform endoscopic cyclophotocoagulation (ECP).
A novel approach to IOP control
BVI reports that the Leos system is supported by emerging data from a randomized controlled trial (RCT), which demonstrated both the safety and efficacy of the device in glaucoma patients. Although full trial results have not yet been published, the company notes that this clinical data contributed directly to the FDA clearance.
The Leos system is intended for use across a wide spectrum of glaucoma types and stages, with particular promise in earlier-stage disease management. More than 76 million people worldwide are currently living with glaucoma, and that number is projected to reach 111.8 million by 2040.1
READ MORE: Open vs Closed Angle Glaucoma: What Are the Differences?
The ab interno, laser-based procedure enabled by Leos offers a new option for lowering IOP by directly treating the ciliary processes responsible for aqueous humor production. The system’s integrated endoscope provides real-time visualization of these structures, which are traditionally difficult to access and image clearly.
“The development of Leos was driven by a deep understanding of the challenges clinicians face in managing glaucoma, such as visualization and access to target tissue,” said Mikhail Boukhny, vice president of Global R&D at BVI. “Our goal was to create a system that not only offers a novel and effective treatment approach but also enhances the surgical experience through improved intuitiveness, precision, and ease of use.”
Key features include:
- A minimally invasive ab interno approach
- Laser ECP treatment targeting aqueous production
- Integrated endoscopic imaging for enhanced visualization
- Designed for ease of use within existing surgical workflows
READ MORE: New Solutions in Glaucoma Management
Future rollout plans
BVI also pointed to recent product approvals in the European Union and Asia, alongside a $1 billion capital raise, as part of its global strategy to scale new technologies across ophthalmic markets.
The company anticipates that Leos will be commercially available in the U.S. later in 2025.
Editor’s note:
- See BVI Medical’s press release on the FDA clearance of Leos for more information.
- This content is intended exclusively for healthcare professionals and eye care practitioners. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
Editor’s Note: This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
Reference
- Tham YC, Li X, Wong T Y, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121(11): 2081–2090.