From topical losartan to magnetic cells, five talks explored evolving corneal therapy options, and the journey to bring them to the patients who need them most.
Laboratory persistence, clinical serendipity and a willingness to fail. That’s what it takes to translate corneal research into practice, according to a Day 3 symposium at the American Academy of Ophthalmology Annual Meeting 2025 (AAO 2025).
The Cornea Society’s Novel Corneal Treatments symposium offered an unvarnished look at how laboratory discoveries become clinical tools.
From a retiring researcher’s three-decade quest to repurpose an antihypertensive drug, to a medalist’s admission that her now-established procedure nearly died in its infancy, the five presentations traced innovation’s messy, non-linear path from hypothesis to operating room.
Killing myofibroblasts to clear scars
Dr. Steven Wilson (USA) opened by announcing his laboratory’s closure and impending retirement in 42 days—but not before sharing three decades of work on topical losartan for corneal fibrosis. The angiotensin II receptor blocker works by inhibiting ERK1/2 phosphorylation in the non-canonical TGF-beta pathway, inducing myofibroblast apoptosis.
“With losartan, after about two weeks, most of the myofibroblasts are gone,” Dr. Wilson explained. “But then the time duration of treatment mandated can be six months in some patients for the corneal fibroblast to come back in, reabsorb all of that extracellular matrix that was disorganized, and also repair the basement membrane.”
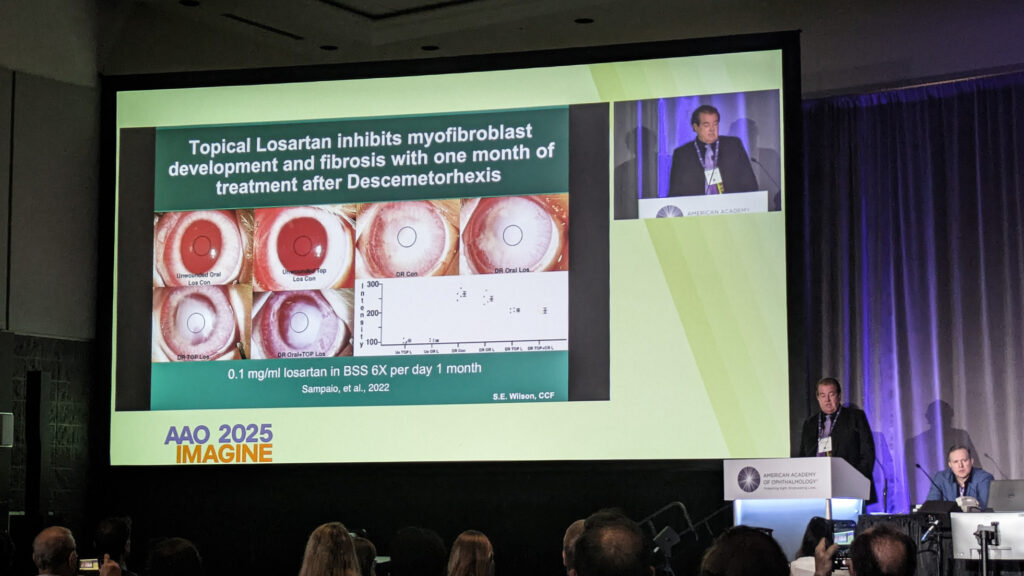

Brazilian cornea specialists quickly adopted the approach off-label, with notable results in herpes simplex scars. Dr. Wilson highlighted a 15-year-old with herpes zoster scarring, treated by colleagues in Brazil, improving from 20/200 to 20/20—particularly significant since the fellow eye was blind from toxoplasmosis.
“Even if the scar is 20 years old, there are active myofibroblasts there, and if you eliminate those, you can eliminate the scar in most cases,” Dr. Wilson noted.
Before concluding his talk, Dr. Wilson imparted some critical dosing guidance: never exceed 0.8 mg/mL with an intact epithelium, as higher concentrations prove epithelial toxicity.
Clinical trials are expected to launch next year in the United States and Europe.
READ MORE: Elevating IVT Injection Precision with FDA-Cleared STERiJECT™ Ophthalmic Low Dead Space Needle
ROCK inhibitors, from bench to bedside
Prof. Shigeru Kinoshita (Japan) traced the two-decade evolution of Rho kinase inhibitors, from their unexpected discovery during embryonic stem cell work to current clinical applications. Early studies revealed the compound promoted monkey endothelial cell adhesion, proliferation and survival—”everything seems to be good for the corneal endothelial cells.”
Preclinical models demonstrated accelerated wound healing in endothelial defects, but Prof. Kinoshita carefully delineated limitations: “Generally speaking, commercially available topical ROCK inhibitors are still not good enough or not so effective as an isolated medical therapy for FED [Fuchs endothelial dystrophy].”

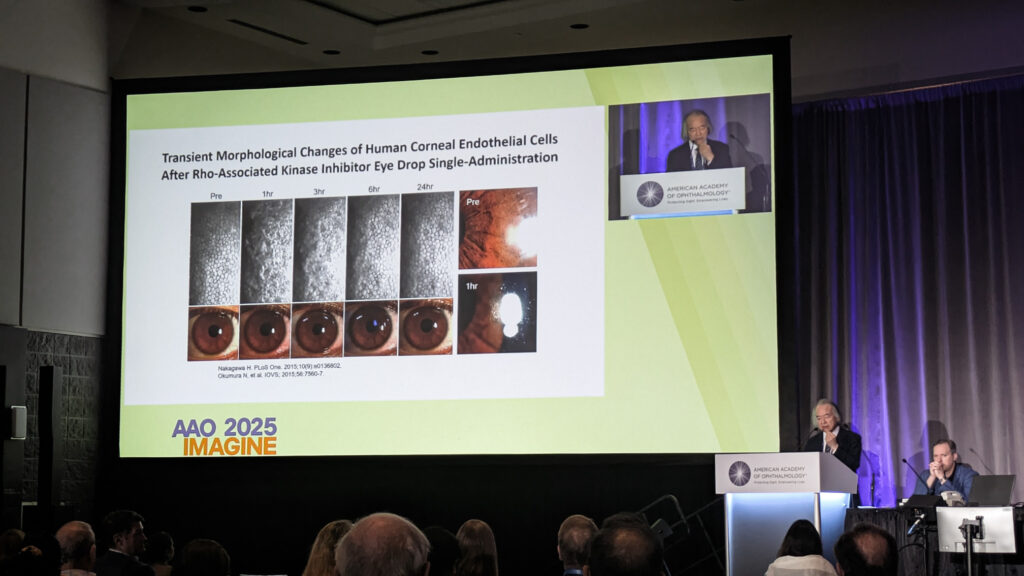
The application most supported by evidence involves combination therapy, particularly post-Descemet membrane endothelial keratoplasty (DMEK). Prof. Kinoshita mentioned work showing ripasudil upregulates pump function markers like aquaporin-1 and SLC4A11.
“ROCK inhibitors are essential to produce a culture of human corneal endothelial cells that maintain healthy mitochondrial function,” he said, noting applications beyond wound healing.
Magnetic cell nanotherapy
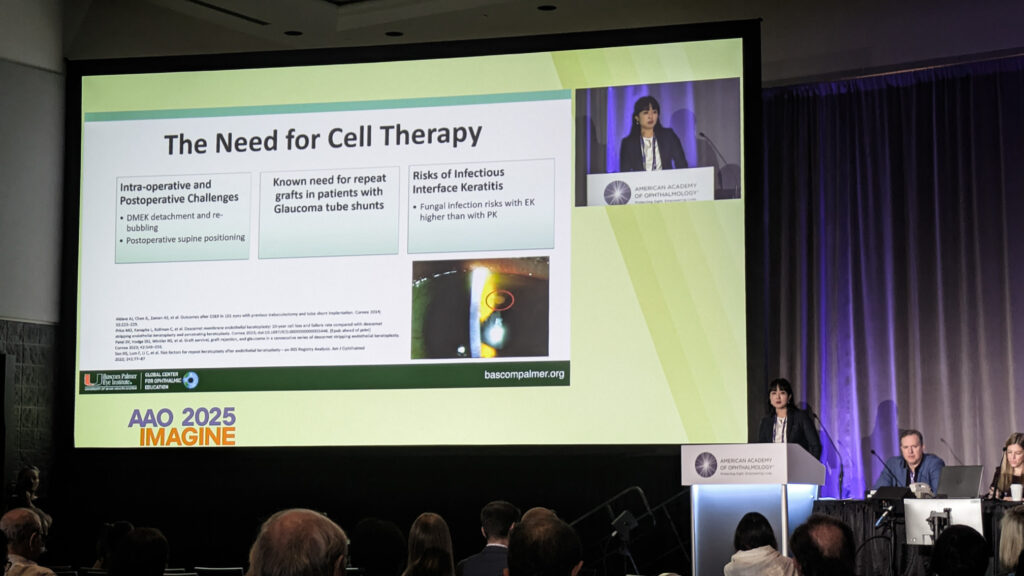
Dr. Ellen Koo (United States) addressed a critical limitation in endothelial cell injection therapy: secondary cell loss from reflux, poor adhesion and apoptosis. Her solution involves magnetic nanoparticles combined with an external eye patch, creating a magnetic field to rapidly integrate cells onto the recipient cornea.
“This video really highlights the efficacy of magnetic cell therapy,” Dr. Koo demonstrated, showing a video. “You can see those cells that are matched to magnetic nanoparticles really adhere to this magnet.”
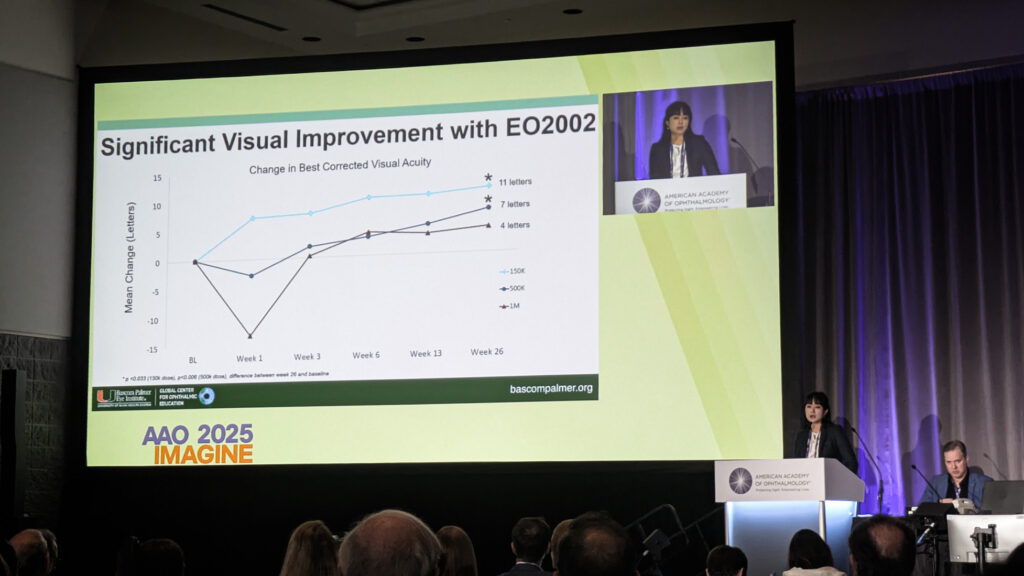
Phase I/II trial results revealed optimal dosing at 150,000 cells, with patients achieving meaningful visual gains. Serial retroillumination photography showed guttae reduction over six months.
“We’re seeing this sustained improvement over time, which really helps us think, ‘What is happening to those cells after injection?’ There must be some modulation of the guttae in a cell-to-cell and cell-to-matrix interaction.”
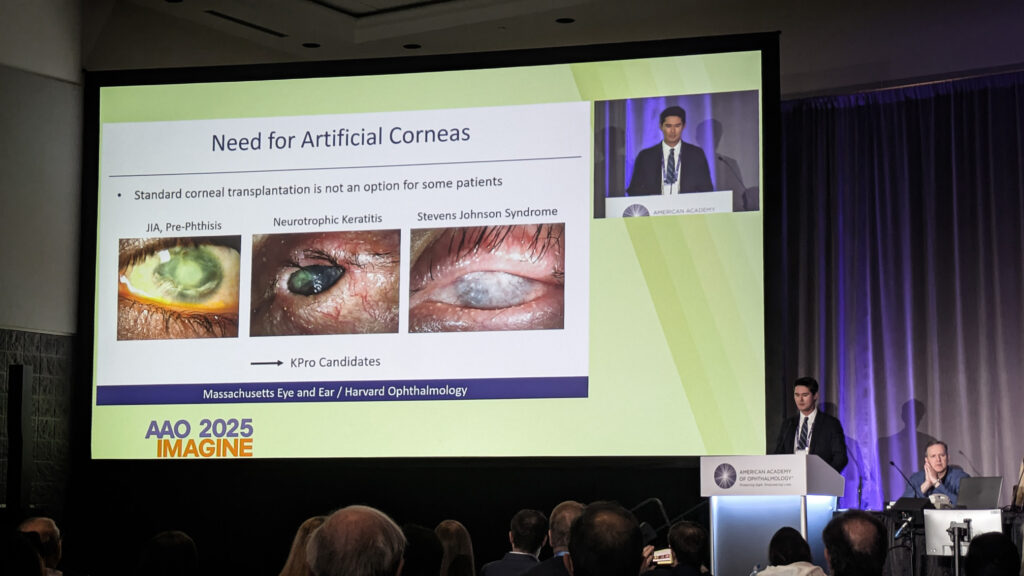
Boston KPro for ‘hopeless’ cases
Dr. Thomas Dohlman (USA) reviewed the Boston keratoprosthesis (KPro) landscape, noting over 21,000 devices implanted worldwide since its 1992 U.S. Food and Drug Administration (FDA) approval.
For failed grafts, autoimmune conditions and limbal stem cell deficiency—cases where standard transplantation fails—KPro offers an alternative. But with artificial devices, the fundamental question differs from standard transplantation.
“Most important is, is the device retained?” Dr. Dohlman said. The answer to his question shows promise for the treatment. “Two-year retention is 87%. Five-year retention is 71%,” said Dr. Dohlman.
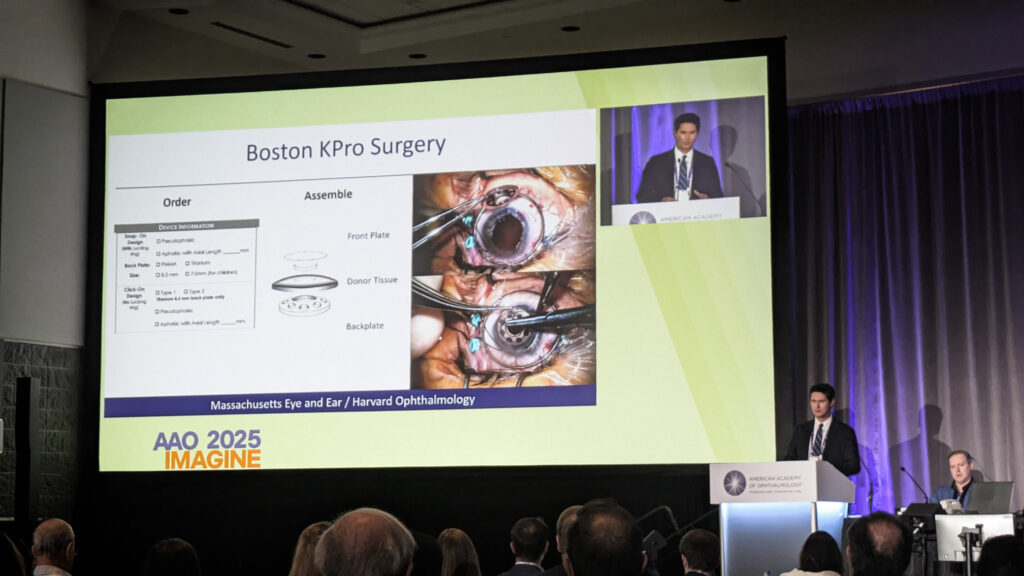
When retained and the posterior segment remains healthy, visual outcomes can be substantial, with 42% achieving 20/200 or better postoperatively versus 21% preoperatively. However, complications remain substantial: retroprosthetic membrane occurred in 51% at five years, with infectious keratitis, endophthalmitis and glaucoma each around 20% to 25%.
Dr. Dohlman also gave an update on ongoing work with tumor necrosis factor (TNF)-alpha inhibition, having obtained an Investigational New Drug (IND) application for adalimumab trials based on animal data showing suppression of retinal ganglion cell apoptosis.
Lower-cost alternatives like the Lucia KPro and fully synthetic devices represent the field’s evolving trajectory.
READ MORE: Pearls Under Pressure: Practical Glaucoma Tips from AAO 2025
The serendipity of DSO
Dr. Kathryn Colby (USA), the 2025 Castroviejo medalist, opened by crediting serendipity alongside scientific rigor.
Her Descemet stripping only (DSO) procedure emerged from connecting two disparate observations: failed DMEK grafts clearing in Fuchs patients and inadvertent rhexis cases with good outcomes.
“I literally had an ‘aha’ moment,” Dr. Colby recalled of a 2012 lecture. “I was sitting in that audience, and I’m like, oh, this has to work.”
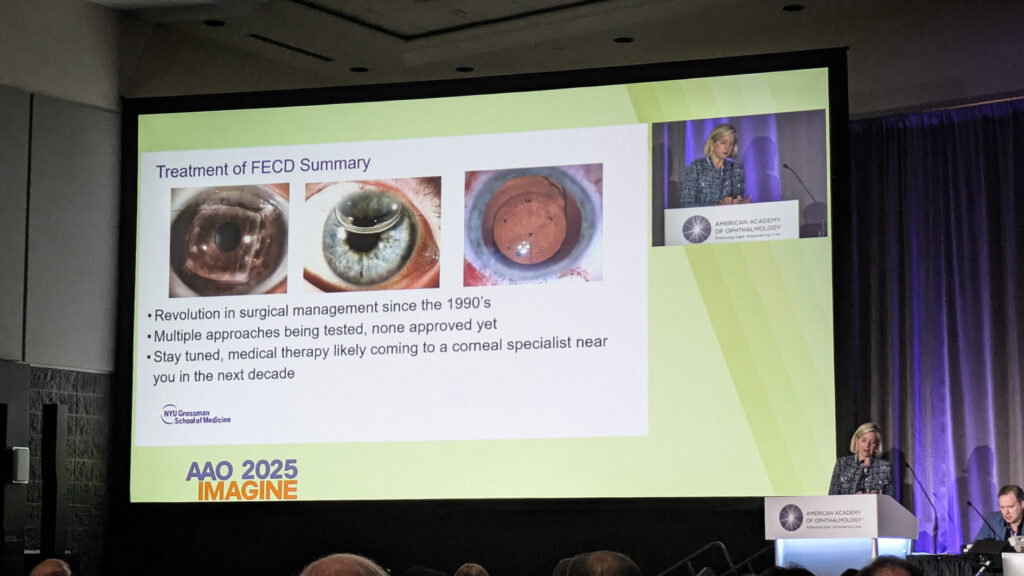

Her index patient—carefully chosen as an attorney who understood informed consent—achieved 20/20 vision at 15 months and has remained stable approaching 12 years.
“You have to have some serendipity because in these early series, about 25% of cases did not clear. Had he not cleared, I would not be standing here talking about this now,” Dr. Colby said, stressing the role of luck.
She detailed Phase III trials combining DSO with ripasudil, expecting final data in 2026 and showcased novel scanning specular microscopy revealing previously invisible peripheral endothelial populations.
Her closing wisdom? “You can’t be afraid to fail. You must have a thick skin…You have to think outside the box and be able to see connections from observations. Make the best decision despite incomplete information.”
Editor’s Note: The American Academy of Ophthalmology Annual Meeting 2025 (AAO 2025) is being held on 17-20 October 2025, in Orlando, Florida. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore
